ISO 15378 is an international standard certification that adds the requirements related to cosmetic/ pharmaceutical packaging materials to the requirements of ISO 9001 (quality management system).
The primary packaging materials for pharmaceuticals include glass, rubber, plastic, aluminum containers/parts, films, foils and coated containers, and these packaging materials come in direct contact with medical products, so it is necessary to comply with safety, efficacy, and reliability requirements.
For cosmetic packaging materials, the quality management system must be established by determining the application level of the ISO 15378 standard requirements and determining the GMP application level based on the risk of cosmetic packaging materials.
- Market demands for safe and reliable containers
- Customer demands for higher level of quality in the manufacturing process
- Accelerate entry into overseas domestic cosmetic and medical product markets
- Strengthen the ability to control and respond to risks such as contamination and manufacturing errors
- Secure external trust and customer satisfaction by strengthening the quality competitiveness
- Reinforcement of competitiveness in overseas export by acquiring international standards
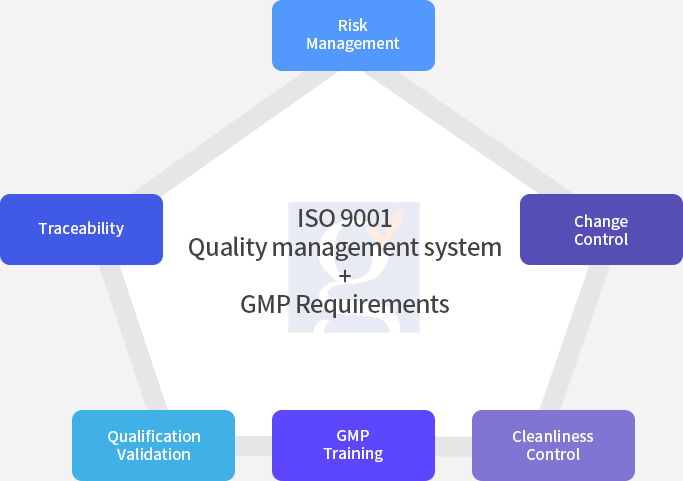

GSC has professional auditors for ISO 15378 certification. We will do our best to grow high-level management systems in organizations through our auditors who has the knowledge of ISO 9001 requirements and GMP regulations.