After completing the OTC registration process and registering as a new manufacturer, the FDA will conduct a Pharmaceutical Standard GMP Audit.
There are two types of FDA OTC audits, and the audit period takes about 5 days per company.
Surveillance inspection
When registered by the FDA, it must be audited once every year or two.
For-cause inspection
This inspection is performed when there is any suspicion about the registration. When complaints from consumers and competitors are received, strict inspections will be performed.
- Essential to maintain US exports
- Being prepared and responsive for the actual FDA audit
- If measures related to the FDA audit is inadequate, it may cause continued difficulties in exporting to the United States
Mock inspection based on the current US FDA requirements is performed, and the analysis of the manufacturing facility’s management status and GAP is performed.
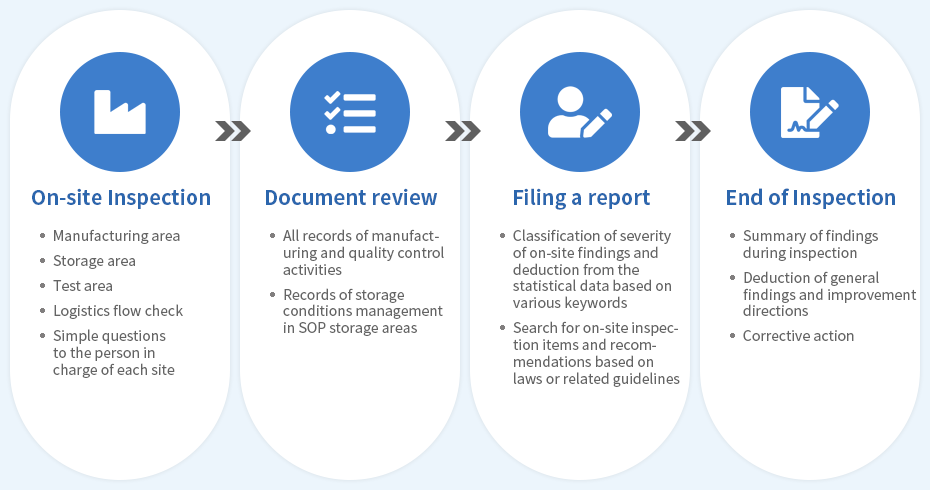
- Detailed design review
- Data on the facility, such as factory floor plan, cleanliness, air conditioning, etc. - Documentation system review
- Documents such as standards (regulations and SOPs) - Equipment qualification review
- Materials related to support, manufacturing, and testing facilities - Validation review
- Validation data on matters, such as process and cleaning

GSC is doing its best to support customers' global corporate activities by accurately evaluating the suitability of products through the latest information and expertise.