Due to the characteristic of medical devices, which is that they directly touch human bodies, all governments regulate the market entrance of medical devices under strict standards. The US manages the medical devices through the FDA.
In the US, under Section 201 (h) of the FD&C law, a medical device is defined as the definitions stated below. Accessories and components, on top of finished products, are considered as medical devices in some cases in the US.
A “medical device” refers to a device, equipment, tool, insert, in vitro reagent, or any object related to or similar to them.
" It includes the following accessories and components.
- An official state diagnosis, American pharmacopoeia, or any record in their revisions made on them
- Something with the purpose of diagnosing the diseases or conditions, reducing treatments, or preventing diseases or conditions of humans or animals
- Something that affects the structure or function of the human or animal body, but does achieve its main purpose not through body chemistry and is not affected by metabolism in order to achieve that purpose
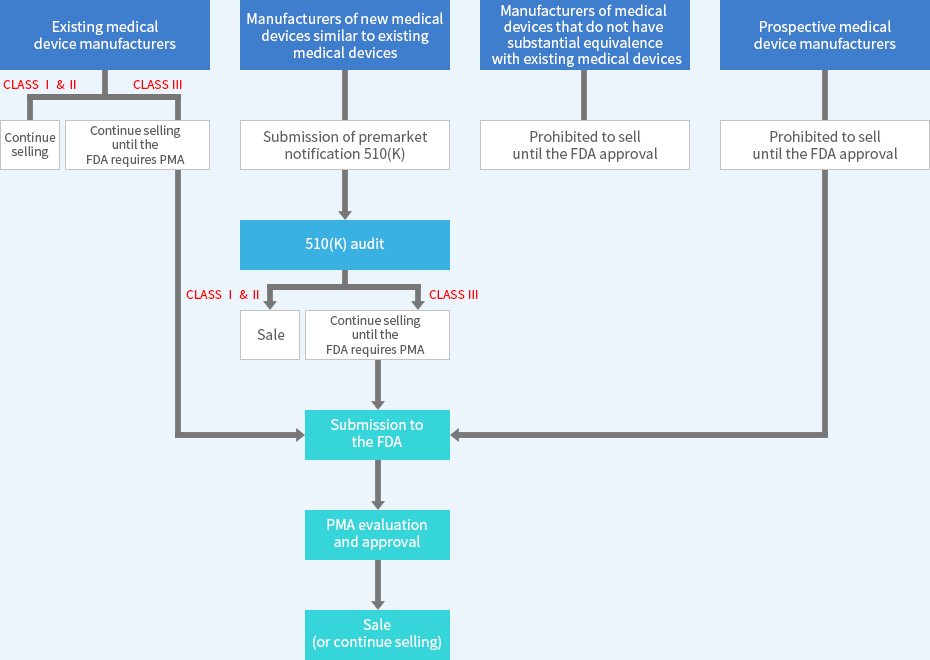
3 Classes of Categorization
- Class I Device : Registration, Device Listing
- Establishment registration
- Device Listing
- Class Ⅱ Device : Registration, Device Listing + 510(k) submission
- 510(k) clearance (PMN - 510(k) certification, equivalence comparison, safety, and efficacy verification)
- Establishment registration
- Device Listing
- Class Ⅲ Device : Registration, Device Listing + PMA
- PMA (Pre-market approval, clinical trial application)
- Establishment registration
- Device Listing
- Essential for entering the US market
- Enhancement of corporate and brand image
- Establishment of product safety and reliability through FDA certification
- New business opportunities
- Establishment Registration: Form 2891 must be filled out and submitted
The FDA mandates registration of factories and facilities not only in the United States but also overseas.
Foreign manufacturers are mandated to register as well as American manufacturers. - Foreign manufacturers must have a resident US Agent in the United States, and the US Agent is responsible for liaising with the FDA based on the understanding of the FDA laws as well as the understanding of the manufacturer or product.
- Medical Devices Listing: Form 2892 must be filled out and submitted, and the medical device manufacturer must register all applicable devices through the US Agent.
※ Unregistered products will be rejected or confiscated during customs clearance
| Labeling | According to the labeling regulations, the method of use, efficacy, and precautions of the product, including packaging, instructions, and advertising text, should be written in English in detail. |
| Notification and repair, exchange, and refund | The manufacturer is obliged to immediately notify the user or repair, exchange or refund any non-conforming medical device found. |
| Records and reports | Manufacturers must keep all test and inspection records to ensure the quality of medical devices, and, if necessary, are obliged to report relevant matters. |
| Medical device restrictions | FDA may restrict the sale, distribution, or use of a medical device if it is impossible to reasonably guarantee the safety and effectiveness of the medical device. |
| Registration and listing submission | Every year, facility registration and a list of medical devices in distribution must be submitted, |

GSC will do our best to satisfy customer needs and FDA regulations for US FDA medical device manufacturing or export licensing through the US FDA U.S Agent that can respond in real time.