ISO13485 standard stipulates the requirements for the system of the organizations that design, develop, produce, install, and provide additional services for medical devices based on ISO 9001 standards and additional standards which specially apply to medical devices.
Establishing a medical device quality management system that can ensure the safety of medical devices is essential for exporting companies.
- Comply with legal regulations related to medical devices and respond to GMP (Medical Device Manufacturing and Quality Control Standards) certification
- The ISO 13485 certification is a prerequisite for companies preparing to enter the medical industry and export overseas
- Maintain a common quality system approach and consistency in the supply system between suppliers and subcontractors
- Product safety can be increased through continuous quality improvement and makes it easy to acquire the CE certification
- Ensure safety and quality for the entire life cycle of medical devices and ensure reliability to customers
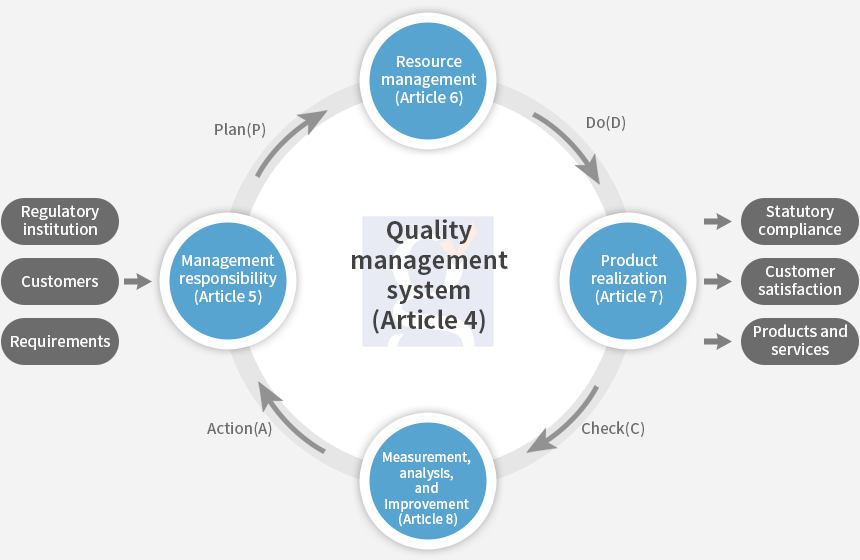

GSC will do our best to ensure continuous growth of organizations by evaluating the suitability of the organizations’ medical device quality management systems through its auditors who have experience in various medical device items.